Nucleus Network has today launched a new campaign “Be a Lifechanger“ aimed at educating Australians about clinical trials, why they are important, what is involved, and why their participation is crucial for the advancement of medicine to improve lives.
The global COVID-19 pandemic has placed medical research in the spotlight like never before. With the world eagerly anticipating a vaccine, public interest, scrutiny and participation in clinical trials has increased exponentially. The impact it has had on our lives has prompted Aussies to step up and become Lifechangers in greater numbers than ever before.
“The entire medical research industry deserves recognition for getting behind the search for a vaccine to COVID-19,” says Cameron Johnson, Nucleus Network CEO. “However, there is one group that is truly irreplaceable, and that is the Australians who sign up for these trials. Without their willingness to participate, we would not be able to advance new medicines for many conditions such as asthma, cancer, diabetes, migraines, osteoporosis and yes, COVID-19.”
Your participation will help someone you know

According to The Australian Institute of Health and Welfare, one out of two Australians suffer from at least one chronic health condition, such as arthritis, asthma, back pain, cancer, cardiovascular disease, diabetes or mental health impairment. Sixty per cent of Australians over the age of 60 suffer from more than one of these conditions. This is in addition to the vulnerability that we all have to infectious diseases that can spread quickly and have severe impact on our daily lives, as COVID-19 has shown.
“Nucleus Network has conducted trials for treatments of many chronic conditions as well as infectious diseases that can greatly impact our society,” says Dr Paul Griffin, Nucleus Network Principal Investigator. “From medicated patches to help Alzheimer’s patients with memory and brain function through to treatments for Motor Neurone Disease (MND) and preventing the next viral outbreak, every single day we understand more and more about how we can fight these diseases.”
“We want people to understand just how much they are helping by participating in trials. As well as being reimbursed for their time they have the satisfaction of knowing that they are helping make a real difference to the lives of many people around the world.”
Safety is our highest priority
Clinical trials are a time-consuming part of the medical research process, but highly necessary and with stringent safety protocols to minimise risk.
“When conducting first-in-human trials, we aren’t going in blind. We already have a substantial amount of data before we even think about bringing people into a study,” says Dr Jason Lickliter, Chief Medical Officer at Nucleus Network. “There is extensive laboratory testing, including detailed studies in animals. By the time it comes to testing on people, we have a fairly accurate picture of the expected effects and safe dosing levels.”
Queensland Biosimilar studies: not a new medicine, just a new recipe

Clinical trials don’t just evaluate new medicines, sometimes it can also be medicines already in the market that are being used in new ways, administered in a different manner or a newly developed process to make a biological drug that is already approved for use. These are called “biosimilar trials”, as they are biologically similar to a drug already available.
“Biosimilars are just as important to us and the community as any new drug research,” says Dr Griffin. “Biosimilar studies give us a diversity of options for treatments, and leads to cost savings for people who need the drug, as well as providing an opportunity to improve treatments already on the market.”
Demystifying the process: What does a clinical trial look like?
“A common question from people thinking about participating in a clinical trial is ‘what is involved?’,” says Dr Griffin. “I think there’s a lot of TV shows and movies that depict medical research as exciting and dramatic, but the truth is that they are mostly calm and even occasionally boring process for our participants.”
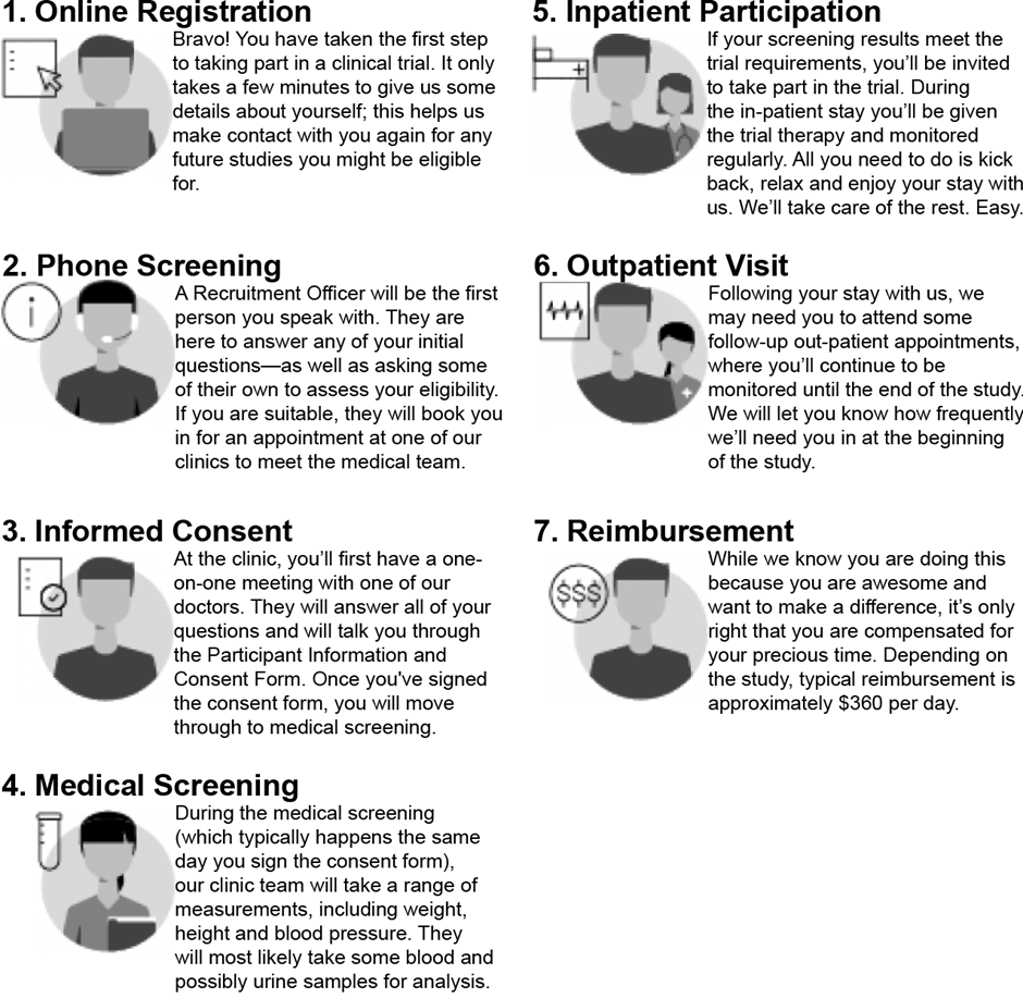
At registration participants are asked an assortment of questions regarding their health, lifestyle and general condition, to understand if there is anything that might affect the results of the study.
After they register, they are matched with any openings in upcoming trials. Some trials might need to study the effects on young, healthy people, some others might target people with specific conditions.
After the initial screening, suitable participants are offered a place in a study. They will need to ensure they can attend the required appointments and will be given detailed information about the study. The appointments might be spread across a long period of time, but each one is important to ensure the clinical trial has accurate results.
If they decide to participate, they then proceed to a medical screening. If they pass this, they are accepted into the participant group.
“We understand that it may be a bit daunting for participants to be in a clinical setting during a trial,” says Dr Griffin. “We try to make it as welcoming as possible. We see participants as partners in this, someone we work with,” he adds.
The end result of these studies is a comprehensive data set that can be provided to pharmaceutical companies and regulatory bodies to determine whether the treatment is ready to be made available to the public or whether it needs to undergo further studies to get a more complete understanding of the effects and safety of it.
The future of clinical trials
As the medical research sector looks beyond COVID-19, one thing is abundantly clear—there are many conditions, illnesses and diseases that can benefit from improved treatment options.
Nucleus Network will continue to help conduct clinical trials for COVID-19 vaccines and treatments, along with many other promising pharmaceuticals that offer improved health outcomes.
Over 25,000 people have participated in more than 1,000 clinical trials with Nucleus Network, without a single loss of life. Hundreds of treatments are now on the market, and millions of people are living with a better quality of life as a result of the contributions of these participants.
Australians can sign up to participate in a trial with Nucleus Network and help us advance medicine and improve lives https://www.nucleusnetwork.com/participate-in-a-trial.
